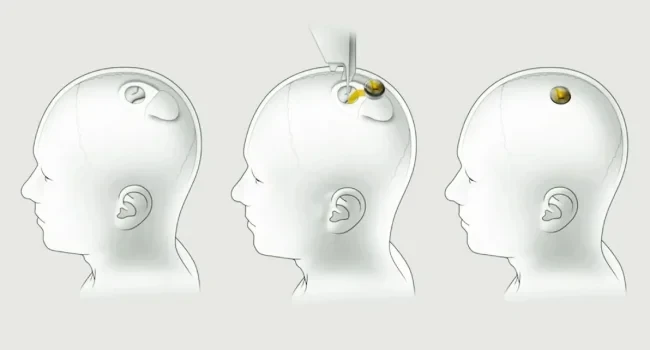
Businessman Elon Musk's company Neuralink reported that during the testing of its neuroimplant installed in the human brain, the wires of the device moved from their original position. At the same time, as notedReuters:, citing its own sources, the company learned about this problem, as it also appeared in animal tests.
Neuralink is testing a neuroimplant that will allow stroke patients to control computers with the power of their minds. In January 2024, the neuroimplant was first placed in the human brain. The first person to participate in the test is Noland Arbo, who had a stroke in 2016. Last week it turned out that the implantsome of the wires are outfrom the patient's brain, resulting in fewer wires to read signals from the brain.
Neuralink did not report any adverse effects of electrode displacement for Arbo, nor did it say how many of the 64 electrodes were displaced. The company only said it was able to restore the neuroimplant's ability to read signals by modifying its algorithm and making the neuroimplant more sensitive.
According to Reuters, Neuralink learned about the problem long before the start of human trials. Animal tests the company conducted last year, before US regulators got approval to test the chip on humans, showed that the neuroimplant's wiring could become dislodged. However, Neuralink considered that this did not pose a major risk and did not change the structure of the neuroimplant.
Reuters interviewees noted that if Neuralink continues testing without changing the device's design, the problem could resurface and become much more significant if more wires are displaced and the implant's sensitivity cannot be increased.
Redesigning a device has its risks. In particular, wiring can damage brain tissue if they become dislodged again or if the company has to remove the device. Neuralink's goal is to ensure that the electrodes are easily removed, allowing the implant to be updated as the technology improves.
By the way, according to one of the sources of Reuters, the US Food and Drug Administration (FDA) was also aware of the problem, because Neuralink provided regulatory authorities with all the results of animal tests. The agency declined to comment on the report, saying only that the FDA will monitor the safety of Neuralink study participants.